The universe, the atom, and a cat both dead and alive: Understanding Quantum Mechanics | Technology News

The transistors that power your smartphone, MRI scanners that detect tumours and blocked blood vessels of the heart, atomic clocks in the GPS that help cars and planes navigate — all work on a science that, for decades, left even the sharpest minds deeply uneasy. Its predictions seemed so bizarre that Albert Einstein, famously known for his love of scientific order, remarked: “God does not play dice with the universe.”
That science is quantum mechanics — the framework that governs the universe at its smallest scales.
At the heart of quantum mechanics lies a radical shift in understanding: Matter and light, when examined at the microscopic level, behave both like particles and waves. Even more strangely, these particles exist in a superposition of all possible states until they are measured or observed. That’s like saying reality settles into a definite state only when you observe it. Sounds exotic? Read on.
A glow that didn’t fit
By the dawn of the 20th century, classical physics was on a roll, seemingly explaining most of the phenomena we saw around us — heat, light, electricity, and motion. But on the sidelines, there were already cracks. The theories of classical physics did not seem to hold good at the atomic and subatomic level. Experiments on blackbody radiation revealed a fatal flaw: hot objects did not glow as predicted, especially at high frequencies.
To fix this “ultraviolet catastrophe,” Max Planck proposed that energy come in discrete packets, or quanta. With this simple assumption — each atom emitting or absorbing only whole multiples of a tiny energy unit — Planck’s formula matched the data perfectly. This launched the quantum age.
Bohr’s atom and wave: Particle duality
In the years that followed, while the Newton-Einstein model gave an understanding of the macroscopic Universe, other scientists were trying to explain the atom. In 1913, Niels Bohr used quanta to explain why atoms emit light only at certain colours: electrons circle the nucleus in specific orbits and jump between them by absorbing or emitting a quantum of light.
Soon afterward, Louis de Broglie suggested that matter—like electrons—also behaves as a wave. Experiments confirmed that electrons can form interference patterns, yet still arrive as individual particles.
Story continues below this ad
Werner Heisenberg then showed that it is fundamentally impossible to know at the same time where exactly a subatomic particle is and how fast it is moving – the famous uncertainty principle. Erwin Schrödinger, in turn, described particles as evolving wavefunctions, giving only the probability of where a particle might be found. So clearly, light and matter both speak a dual language: sometimes wave, sometimes particle.
Bohr vs Einstein: A battle of worldviews
But this strange new theory did not sit well with everyone. Albert Einstein firmly believed in an objective reality — one that exists independently of observation. Niels Bohr, however, argued that quantum mechanics fully described nature, even if it defied classical intuition.
Their famous debates came to a head at the 1927 Solvay Conference, where the world’s greatest physicists gathered to discuss the meaning of quantum mechanics. Einstein kept challenging quantum ideas with thought experiments; Bohr, always patient, parried them masterfully. That intellectual duel remains one of the most important scientific conversations of the 20th century.
Schrödinger’s cat: Dead or alive?
Two concepts of the unveiling quantum world especially spooked its critics:
Story continues below this ad
📍superposition, that says a particle can exist in multiple states simultaneously until it is measured, and
📍entanglement, that says two linked particles act in tandem, instantly affecting each other’s state, even if they are far apart.
In 1935, Einstein, Boris Podolsky, and Nathan Rosen (EPR) argued that quantum mechanics was incomplete in describing physical reality. In response, Schrödinger proposed a now-celebrated thought experiment to show why it is absurd to apply the quantum world at the macro level.
A cat is sealed in a box with a radioactive atom, a Geiger counter to detect the radioactivity and a vial of poison. If the atom decays, the Geiger counter triggers and breaks the vial, and the cat dies. Here, if the interpretation of quantum superposition were to be taken literally, the cat would be both alive and dead until we open the box and look.
Story continues below this ad
Schrödinger’s cat became the most enduring image of how the classical world differs from the quantum world. What applies across the macroscopic universe does not apply to its smallest building blocks, and vice versa.
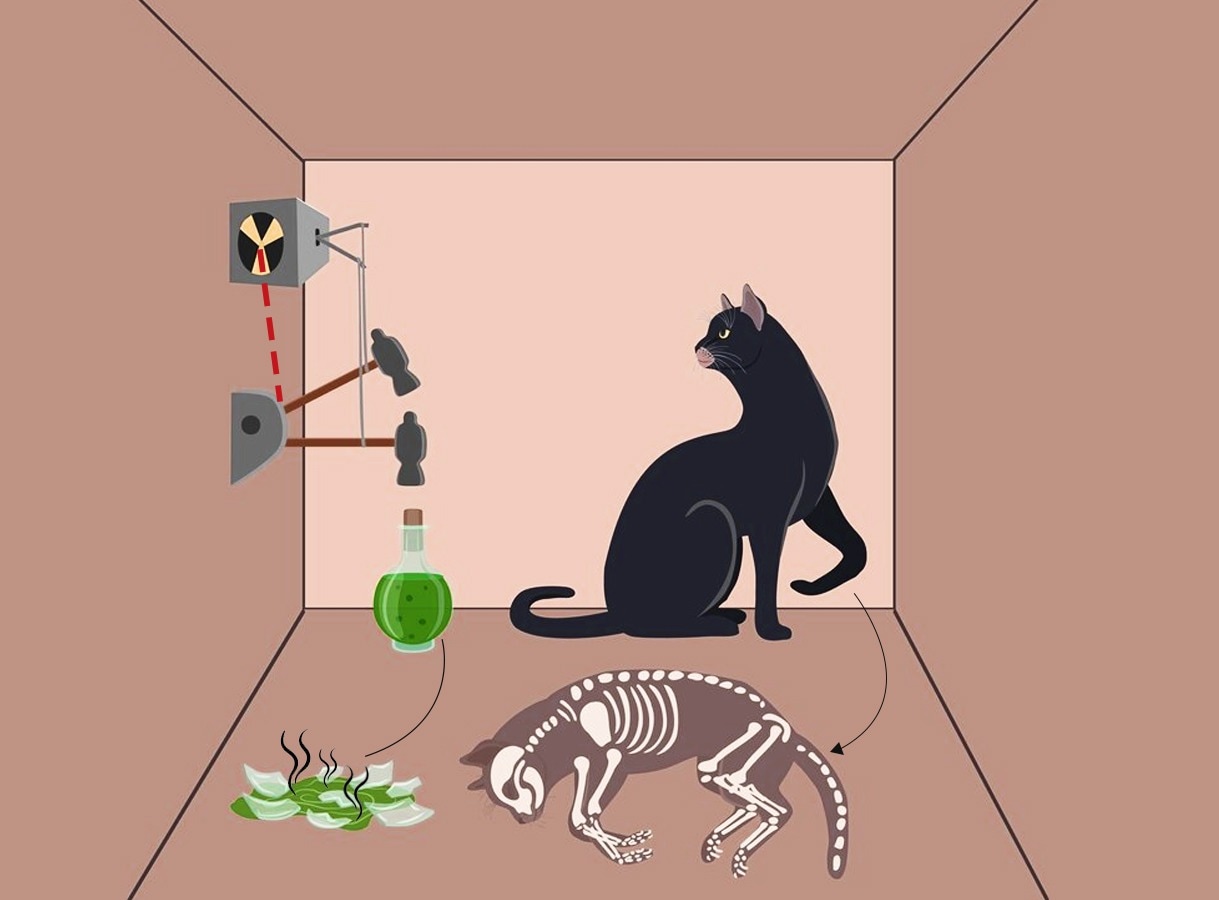 Inside the quantum box: Until observed, the cat is both alive and dead, a paradox that challenges how we understand reality.
Inside the quantum box: Until observed, the cat is both alive and dead, a paradox that challenges how we understand reality.
Bell’s test
The idea that quantum particles are governed by probabilistic rules was deeply unsettling for many physicists who believed nature must be deterministic and objective. To reconcile quantum predictions with the belief in an underlying, well-defined reality, some proposed hidden-variable theories, which proposed that particles in fact possess definite properties at all times, but these are governed by variables that are hidden from current experiments. Because these hidden variables could not be measured explicitly by experiments, the particles which obeyed these laws would appear to behave probabilistically.
In 1964, John Bell devised a practical test. He showed that any local hidden-variable theory must satisfy certain statistical bounds — Bell’s inequalities — whereas quantum mechanics predicts clear violations. Landmark experiments beginning in the 1970s measured entangled particles and found exactly those violations. The result was inescapable: no local, predetermined account can match quantum predictions. Nature truly is non-deterministic and non-local at the quantum level.
From crisis to cornerstone to everyday
From Planck’s first quantum in 1900 to Bell’s experiments in the 1980s, it took decades to accept a world where particles can be waves, outcomes are fundamentally probabilistic, and distant particles can remain mysteriously linked. Yet every test confirmed that quantum mechanics, strange as it is, gives the most accurate description of nature we have.
Story continues below this ad
Once settled, quantum mechanics became the foundation for the technologies we take for granted. Semiconductors rely on quantum energy levels to control electricity. Lasers exploit stimulated emission of photons. MRI scanners use quantum spin of atomic nuclei to create detailed images. Atomic clocks measure vibrations of electrons to keep time with incredible precision.
A final reflection
The quantum revolution shows that moments of crisis, when experiments clash with established ideas, can lead to paradigm shifts that reshape our world. By embracing quanta, waves, uncertainty, and entanglement, we’ve unlocked technologies unimaginable to Planck, Bohr, and Einstein. As two more epic technologies — quantum computing and secure quantum communication — emerge, we continue to turn nature’s strangeness to our advantage. The universe, in all its paradoxes, is also our greatest inspiration.




